Global Experts Group • Agorà 2025 Congress
From Market Chaos to Scientific Clarity
Evidence-Based Comparative Scientific Review of Collagen Stimulating Injectables
Author: Dr. Ghofran Mahmoud | MD Physician | R&D Director
A rigorous evaluation of injectable agents claiming to stimulate collagen, elastin, and cellular regeneration.
Introduction: Separating Market Claims from Evidence
In the current aesthetic landscape, market claims do not always equal evidence. This review was designed to separate biological mechanisms from clinical outcomes, providing a quantified look at dermal changes and safety across various modalities.
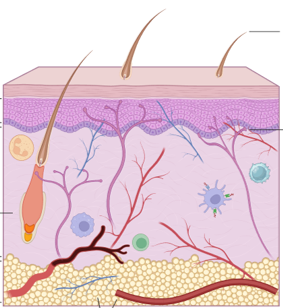
Targeting the Dermis: Fibroblasts, Pericytes, and Macrophages.
Rigorous PRISMA Methodology
To ensure the highest scientific integrity, the review followed a strict PRISMA identification process:
- 780 records identified across PubMed, Embase, Scopus, and Cochrane.
- 196 articles assessed for full-text eligibility.
- 72 studies ultimately included in the quantitative evaluation, ranging from RCTs to histological and imaging-based studies.
Mechanism Groups: How Do They Stimulate?
The review categorizes stimulators into five distinct mechanism groups:
- Scaffold-mediated (PLLA, CaHA, PCL): Particles trigger macrophages to release TGF-β, leading to collagen synthesis.
- DNA-based signaling (PDRN): Activates A2A receptors to increase VEGF and fibroblast proliferation
- Paracrine vesicles (Exosomes): miRNA delivery upregulates fibroblast genes (COL1A1).
- Peptide signaling (GHK-Cu): Modulates MMPs and stimulates fibroblasts.
- Micro-injury: Remodeling via IL-1 and PDGF release.
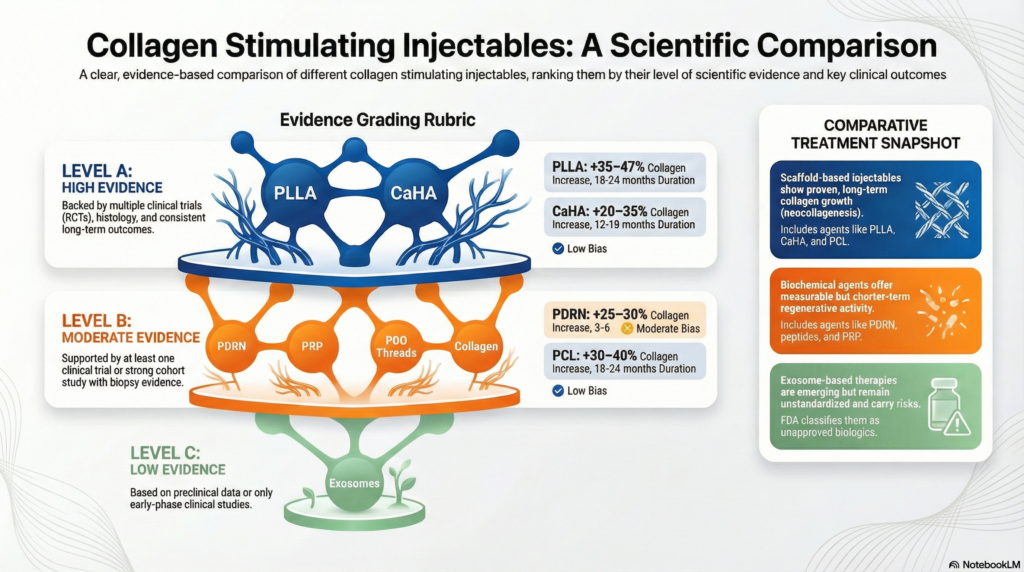
The Comparative Evidence Table
Data suggests that while many agents offer regenerative activity, the duration and evidence levels vary significantly.
| Active Agent | Collagen/Dermal ↑ | Duration | Evidence Level |
|---|---|---|---|
| PLLA | +35–47% | 18–24 Months | High (A) |
| CaHA | +20–35% | 12–18 Months | High (A) |
| PCL | +30–40% | 18–24 Months | High (B) |
| PDRN | +25–30% | 3–6 Months | Medium (B) |
| Exosomes | +20–25% (Elasticity) | 3–6 Months | Medium (C) |
Safety and Ethical Considerations
Safety is the primary mandate. While established scaffolds like PLLA and CaHA have adverse event rates between 1.0–2.5%, emerging cell-derived products face stricter scrutiny. The FDA and EMA currently classify uncharacterized human-source exosome products as Unapproved Biologics . The core ethical principle remains: “Do not inject what you cannot characterize” .
Analogy for Understanding: Imagine your skin is a building that has lost its structural integrity. Biochemical agents (like PDRN or Peptides) are like specialized repair crews that do a fantastic job fixing the surfaces, but they leave after the shift ends. Scaffold-mediated injectables (like PLLA or CaHA) are like installing a permanent steel frame; they provide the long-term support that keeps the building standing strong for years.
Summary: The Future of Regeneration
The future of aesthetic medicine lies in multimodal, evidence-led protocols. While scaffolds provide proven structural neocollagenesis, biochemical agents offer measurable regenerative activity. Combining these approaches ethically and scientifically represents the true path to regeneration.
Join the Academy at Global Experts


